Abstract
Background: An ex vivo photodepletion method has been developed to produce ATIR101 (Kiadis Pharma), a donor lymphocyte infusion (2.0 × 106 cells/kg) administered after haploidentical allogeneic hematopoietic stem cell transplantation (haplo-HSCT) to aid immune reconstitution. ATIR101 is depleted of alloreactive T-cells and early administration after T-cell-depleted haplo-HSCT has the potential to reduce serious complications resulting from delayed immune reconstitution, such as infections, malignant relapse, and severe graft-versus-host disease (GVHD) in the recipient. The safety and efficacy of a single dose of ATIR101 are presented here in a pooled analysis of two phase II clinical trials: CR-AIR-007 (NCT01794299) & CR-AIR-008 (NCT02500550).
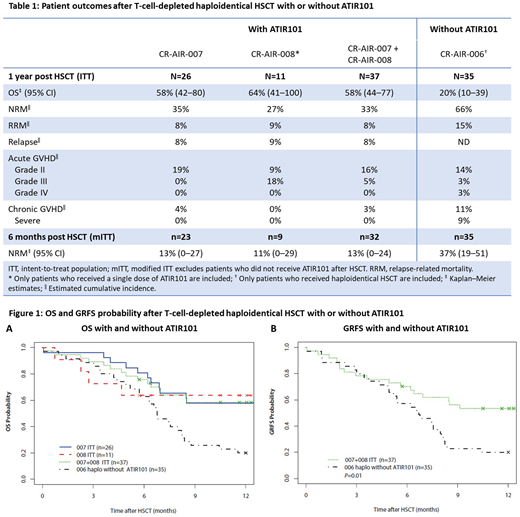
Methods: A pooled analysis of 37 adult patients who received haplo-HSCT. All but 3 patients have completed 1 year of follow-up (data cut-off: June 1, 2018). The study designs are aligned, with similar inclusion and exclusion criteria and baseline patient characteristics. Overall, 32 patients received a single dose of ATIR101; n=5 did not receive ATIR101 and discontinued after HSCT due to death (n=2), graft failure (n=1), and rejection of the ATIR101 batch (n=2). In the pooled cohort, n=24 had acute myeloid leukemia (65%), n=10 had acute lymphoblastic leukemia (27%), and n=3 had myelodysplastic syndromes (8%), with 57% of patients having an intermediate disease risk index (DRI) and 43% a high DRI. All patients underwent myeloablative conditioning followed by a CD34+ selected stem cell graft from a haploidentical family donor. ATIR101, prepared from the same donor, was given at a median of 28 days post HSCT as a single dose of 2.0 × 106 cells/kg, without the use of prophylactic immunosuppression. These data were compared with outcomes from a historic control group of 35 patients treated with a T-cell-depleted CD34+ selected haplo-HSCT without ATIR101 at overlapping participating hospitals in a prospectively planned registry study (CR-AIR-006, NCT02188290).
Results: Thirty-six patients engrafted, with neutrophil engraftment at a median of 14 days (range 8-34) and platelet engraftment at a median of 12 days (range 7-35) post HSCT. Outcomes are shown in Table 1. In the pooled cohort, non-relapse mortality (NRM) was 33% and relapse-related mortality was 8%, compared with 66% and 15%, respectively, in the historic control group. The overall survival (OS) of patients was 58% in the pooled cohort (Figure 1A) and 20% in the historic control group. In the pooled cohort, total chronic GVHD was 3%, chronic severe GVHD was 0%, and acute GVHD grade III-IV was 5%, with 0% grade IV; in the historic control group, the results for total chronic GVHD, chronic severe GVHD, and acute GVHD grade III-IV were 11%, 9%, and 6%, respectively. The Kaplan-Meier curve for survival without grade III/IV acute GVHD, chronic GVHD requiring systemic treatment, or relapse (GRFS) showed significant separation for the pooled cohort versus the historic control group (P=0.01; Figure 1B); the GRFS rates for the two groups were 53% and 20%, respectively.
Conclusion: OS was nearly threefold higher in the pooled cohort than in patients not receiving ATIR101 after haplo-HSCT in the historic control group. These outcomes were achieved without the need for concomitant high-dose immunosuppressive therapy. Disease relapse was limited and NRM in the pooled cohort was half that of the historic control group. Administration of a single dose of ATIR101 after a T-cell-depleted haplo-HSCT was well tolerated. The rate of GVHD is lower in the pooled cohort than the historic control group, suggesting that ATIR101 does not increase GVHD beyond the levels reported with a T-cell-depleted CD34+ selected HSCT alone. GRFS in the pooled cohort was >2.5-fold higher than the historic control group and also seems higher than GRFS rates previously reported for other studies using post-transplant cyclophosphamide (PTCy) after T-cell-replete HSCT (Solh 2017, McCurdy 2017). To further investigate the therapeutic potential of ATIR101, a large, phase III, randomized control trial is currently underway to assess the relative safety and efficacy of ATIR101 after T-cell-depleted haplo-HSCT, versus PTCy after T-cell-replete haplo-HSCT (CR-AIR-009 HATCY; NCT02999854).
Roy:University of Montreal: Patents & Royalties: Author on patent; Kiadis Pharma: Other: Travel support; Hopital Maisonneuve Rosemont: Patents & Royalties: Author on patent. Beguin:Kiadis Pharma: Consultancy. Selleslag:Kiadis Pharma: Other: Financial support for study-related issues. Wagner:Medac: Other: Travel grant. Bonig:Kiadis Pharma: Consultancy. Mielke:Kiadis Pharma: Other: Travel grants, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal